Products Descriptions
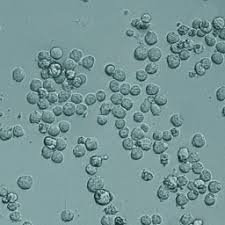
Intraclone ClonaMab is a cutting-edge, chemically defined Animal component free (AOF) cell culture medium specifically engineered to boost the performance of fed-batch processes for producing monoclonal antibodies (mAbs). This innovative formulation is designed to support the high cell densities and prolonged culture durations necessary to maximize product yield and quality, all while reducing the total cost of goods (COGS).
Product Characteristics
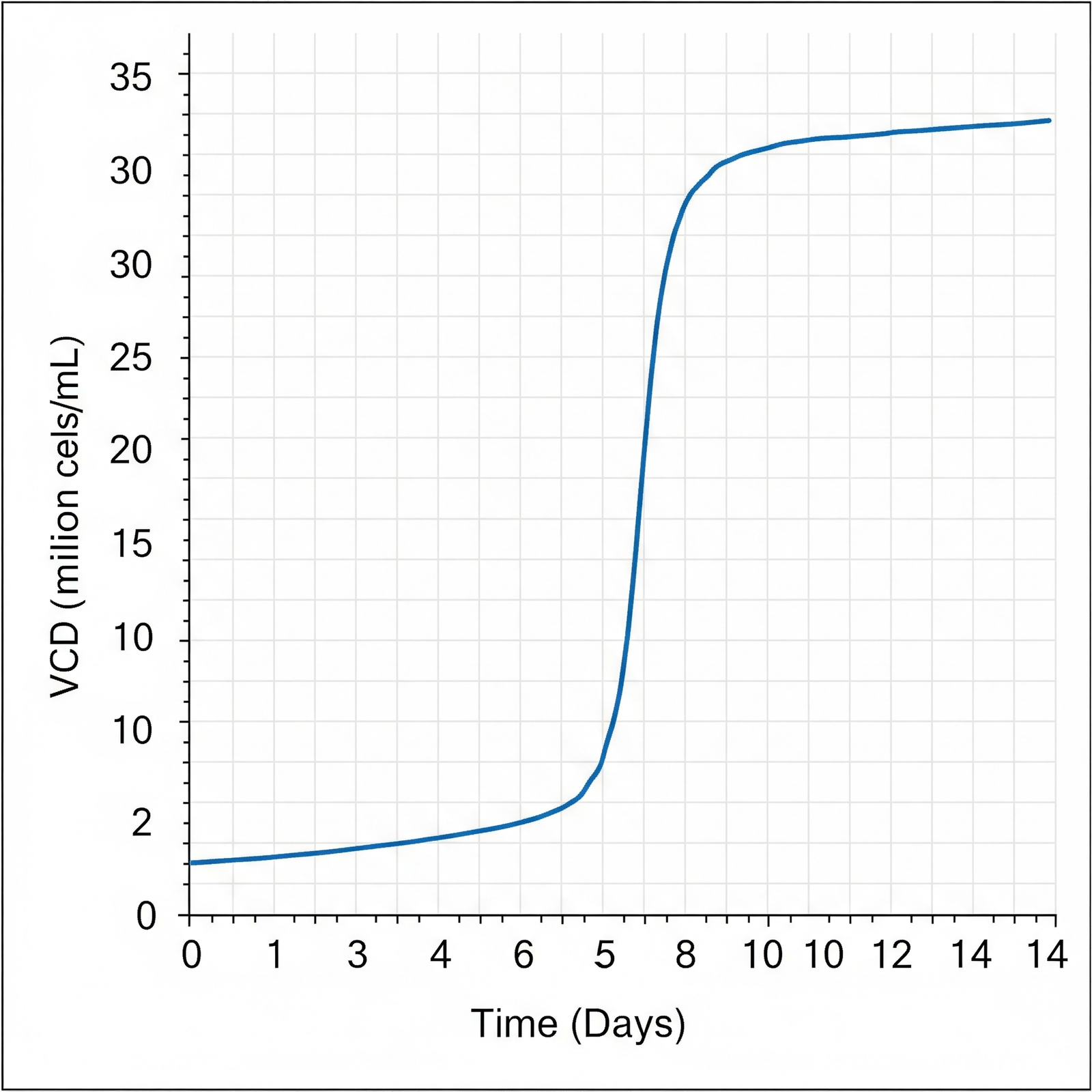
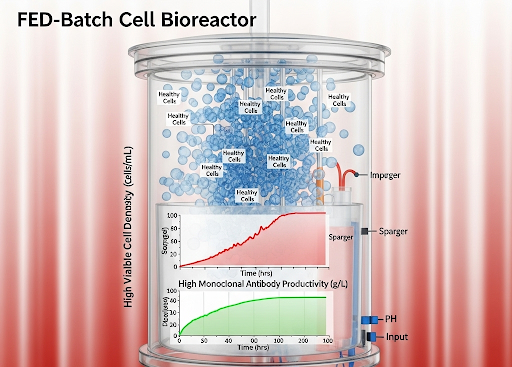
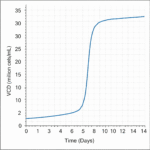
- High Cell Density: Supports cell populations exceeding 20 million cells/mL, enabling significantly increased volumetric productivity.
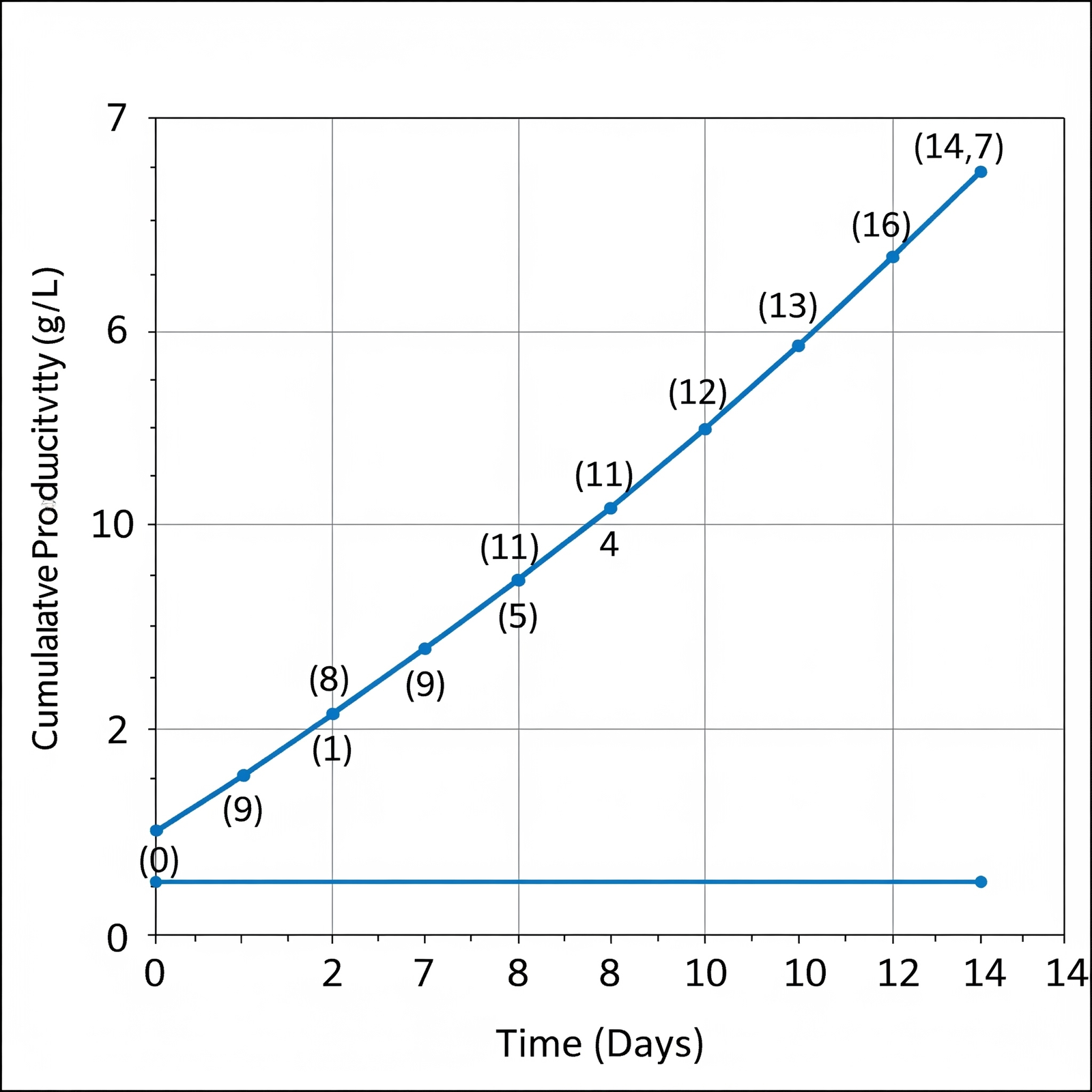
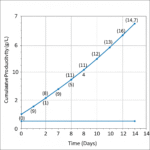
- Superior Productivity: Achieves impressive titers of ranging 5-10 g/L, directly translating to higher product yields.
- Low Ammonia Production: Minimizes the accumulation of toxic byproducts like ammonia, which can inhibit cell growth and impact product quality.
- Low Product Heterogeneity: Promotes consistent post-translational modifications, leading to a more uniform and high-quality final product.
- Chemically Defined Formulation: Ensures lot-to-lot consistency, eliminates the risk of animal-derived contaminants, and simplifies regulatory approval.
- Broad Cell Line Compatibility: Optimized for a wide range of cell types, including CHO, HEK, and insect cells.
- Versatile Application: Suitable for various stages of bioprocessing, from cloning and cell banking to clinical and commercial-scale batches.
- Enhanced Robustness: Improves the overall consistency and reliability of biopharmaceutical manufacturing processes.
Product Overview
Addressing Biopharma Bottlenecks
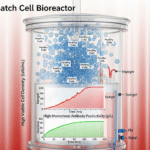
The biopharmaceutical industry, particularly in monoclonal antibody (mAb) production, faces persistent challenges. Current bioprocessing methods often struggle with achieving high volumetric productivity and maintaining product quality, leading to high manufacturing costs and complex downstream purification processes.
One major bottleneck is the limitation of traditional media in supporting high cell densities over extended culture periods. As cell density increases, a fed-batch system must be carefully managed to avoid nutrient depletion and the buildup of inhibitory metabolites, such as lactate and ammonia. High levels of these byproducts can reduce cell viability, shorten the productive phase of the culture, and negatively impact the quality of the secreted mAbs, including their glycosylation patterns and aggregation levels. This product heterogeneity can complicate downstream purification and lead to regulatory scrutiny.
Intraclone ClonaMab addresses these bottlenecks head-on. Its proprietary, chemically defined formulation is meticulously balanced to support a healthy and highly productive cell culture environment. By enabling cell densities > 20 million cells/mL and a prolonged culture duration, the medium significantly increases the total antibody produced per batch. The formulation’s design also focuses on minimizing metabolic stress, which is reflected in its low ammonia production. This not only extends the culture’s viable phase but also contributes to low product heterogeneity, ensuring a more consistent and high-quality mAb product.
The use of Intraclone ClonaMab allows manufacturers to reduce COGS by maximizing the output from each bioreactor run and simplifying downstream processing. Its a versatile and robust solution that offers enhanced performance and consistency across the entire biomanufacturing workflow, from early-stage development to large-scale commercial production.
How to Use It
- Preparation: Intraclone ClonaMab is supplied as a sterile, ready-to-use liquid medium. It’s recommended to warm the medium to room temperature or 37°C before use. Avoid prolonged exposure to light and heat.
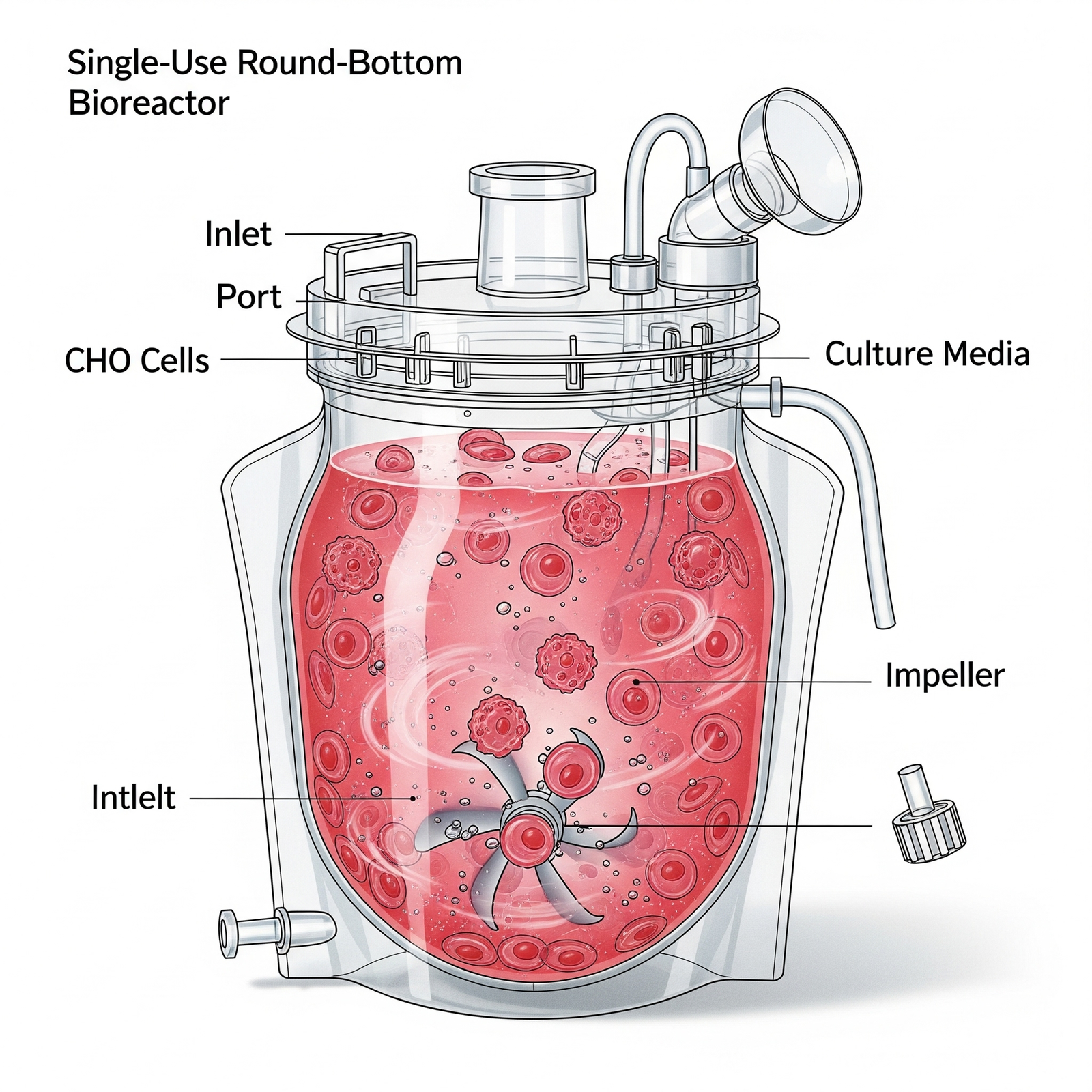
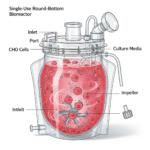
- Cell Culture: Inoculate your chosen cell line (e.g., CHO, HEK) into the Intraclone ClonaMab basal medium.
- Fed-Batch Strategy: Begin the feeding phase typically after the exponential growth phase has started, following your established protocol. The feed volumes and timing should be optimized for your specific cell line and process.
- Monitoring: Regularly monitor key parameters, including viable cell density (VCD), viability, pH, dissolved oxygen (DO), and metabolite concentrations (e.g., glucose, lactate, ammonia).
- Harvesting: Harvest the culture when cell viability begins to decline or when the target productivity is reached, as determined by your process.
For Research Use Only. Not for use in diagnostics procedures, not human or Animal use
Applications
Intraclone ClonaMab is an ideal solution for a variety of applications in the life sciences and biopharmaceutical industries.
- Monoclonal Antibody (mAb) Production: Primary use for maximizing the yield and quality of mAbs in fed-batch and perfusion culture systems.
- Recombinant Protein Expression: Supports high-density growth of various cell lines for the production of other recombinant proteins.
- Cell Line Development: Can be used during the cloning and selection stages to identify and establish robust, high-producing cell lines.
- Cell Banking: Ensures consistent performance and viability for master and working cell banks, providing a stable foundation for future manufacturing runs.
- Process Development and Optimization: An excellent tool for optimizing fed-batch strategies, including feeding regimens and process parameters, to achieve peak performance.
- Therapeutic and Biosimilar Manufacturing: Its robust and consistent performance makes it suitable for both clinical-grade manufacturing and large-scale commercial production of biopharmaceuticals.
For Research Use Only. Not for use in diagnostics procedures, Not human or Animal use